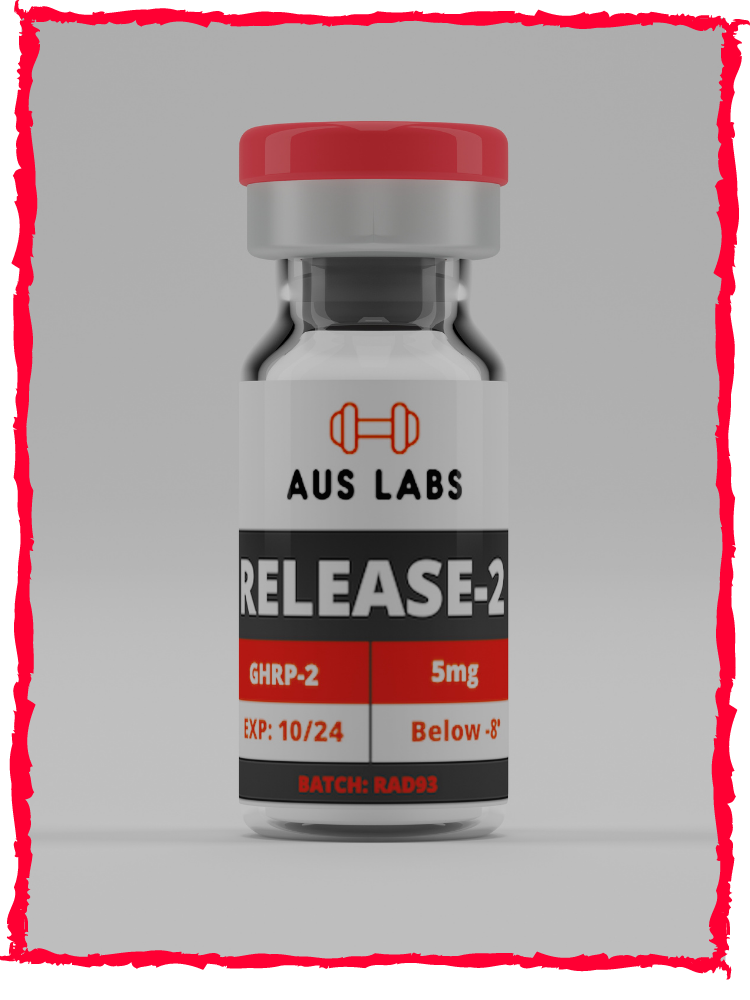
Description
RELEASE (GHRP-2) is an injectable peptide in the category of growth hormone releasing peptides, or GHRP’s. The most common use of these peptides is to increase GH production. Other peptides in this category include GHRP-6, hexarelin, and ipamorelin. With regard to increasing GH, all of these work similarly, and there is no need or advantage to combining them. Instead, the one most suited for the particular case is chosen.
RELEASE (GHRP-2) and all GHRP’s are mimetics of ghrelin, a hormone produced by cells of the stomach in response to a fasted condition, including brief fasts. Ghrelin and ghrelin mimetics work by activating the ghrelin receptor, also called the growth hormone secretagogue receptor (GHS-R1a). Elevated ghrelin levels act towards increasing GH levels by stimulation of ghrelin receptors in the pituitary.
Injection of RELEASE (GHRP-2) or any GHRP stimulates GH release in essentially the same way as fasting-induced elevation of ghrelin levels. The effect can be markedly greater, however levels of GH are easily achieved with proper dosing of any GHRP.
The principal use of RELEASE (GHRP-2) is to provide increased GH levels, which also results in increased IGF-1 levels. This aids fat loss and in some instances aids muscle gain as well. Generally, GHRP use is chosen as an alternate to HGH use, and only rarely is combined with HGH.
Dosage
ALL REFERENCES OF USE ARE FOR RESEARCH SUBJECTS AND NOT HUMANS
RELEASE (GHRP-2) is sold in small vials of 5 mg, which should be stored under refrigeration. (It is acceptable however for them to be mailed unrefrigerated.) The vial is diluted with a convenient volume of sterile or bacteriostatic water. For example, the vial might be diluted with 2.5 mL of water, yielding a solution of 2 mg/mL (2000 mcg/mL.) After the water addition, the vial again will be stored under refrigeration.
When dosing, an appropriate volume will be drawn from the vial with (typically) an insulin syringe, according to the desired dose and the concentration of the preparation. In the above example, a 100 mcg dose would require only 0.05 mL, or “5 IU” as marked on an insulin syringe. A 300 mcg would require 0.15 mL, or “15 IU” as marked on an insulin syringe.
Injection may be subcutaneous, intramuscular, or intravenous according to personal preference.
Dosing will ordinarily be at least twice per day and preferably 3x/day for best effect, taken at least 30-60 minutes before a meal and at a time of non-elevated blood sugar (in other words, after blood sugar has had time to fall since the most recent meal.) The amount taken generally will be from 50-300 mcg at a time.
For increase in GH levels, higher doses within the suggested range definitely increase effect. With regard to healing benefit, for example for tendonitis, the low end of the range is often entirely sufficient and noticeably greater effect is not necessarily seen with increased dose.
While there is no sharp cut-off between a solution of RELEASE (GHRP-2) still being good and having lost potency with time, as a general guideline, a vial should be used within a month of having been reconstituted. Past this, I would discard the vial and start a new one.
Ingredients
RELEASE: 5mg of L-histidyl-D-tryptophyl-L-alanyl-L-tryptophyl-D-phenylalanyl-L-Lysinamide
Storage
Below -20 degrees or the freezer. Once reconstituted -8 degrees or the fridge is acceptable.
Medical References
- Hu, R, Wang, Z, Peng, Q, Zou, H, Wang, H, Yu, X, Jing, X, Wang, Y, Cao, B, Bao, S, Zhang, W, Zhao, S, Ji, H, Kong, X & Niu, Q 2016, ‘Effects of GHRP-2 and Cysteamine Administration on Growth Performance, Somatotropic Axis Hormone and Muscle Protein Deposition in Yaks (Bos grunniens) with Growth Retardation’, PLoS ONE, vol. 11, no. 2, pp. 1–19
- Lee, HG, Choi, YJ, Lee, SR, Kuwayama, H, Hidari, H & You, SK 2005, ‘Effects of dietary protein and growth hormone-releasing peptide (GHRP-2) on plasma IGF-1 and IGFBPs in Holstein steers’, Domestic Animal Endocrinology, vol. 28, no. 2, pp. 134–146
No statements made on this page have been evaluated by the Bundesministerium für Gesundheit (BMG) or the Therapeutic Goods Administration (TGA). These products are not intended to treat, cure or prevent any disease or illness. All products are strictly for research purposes only. Please refer to the TERMS OF USE for more information.